Zero-order elimination kinetics : “Elimination of a constant quantity per time unit of the drug quantity present in the organism.”
First order elimination kinetics : “Elimination of a constant fraction per time unit of the drug quantity present in the organism. The elimination is proportional to the drug concentration.”
Description
Zero-order elimination kinetics :
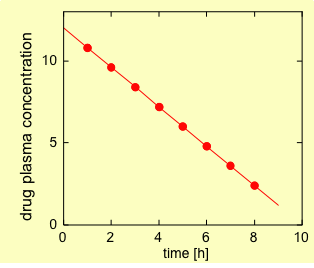
The plasma concentration – time profile during the elimination phase is linear (Fig. 1). For example 1.2 mg are eliminated every hour, independently of the drug concentration in the body.
Order 0 elimination is rather rare, mostly occurring when the elimination system is saturated. An example is the elimination of Ethanol.
First-order elimination kinetics :
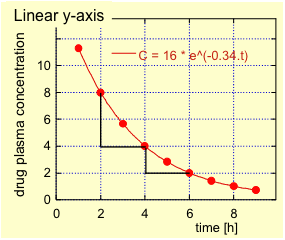
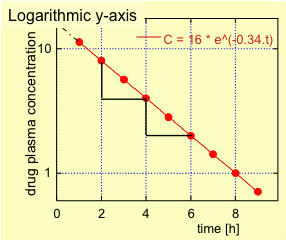
For first order elimination, the plasma concentration – time profile during the elimination phase shows an exponential decrease in the plot with linear axes (Fig. 2.) and is linear if plotted on a semi-logarithmic plot (plasma concentration on logarithmic axis and time on linear axis; Fig. 3.).
For example, 1% of the drug quantity is eliminated per minute. Many drugs are eliminated by first order kinetics.
The time course of the decrease of the drug concentration in the plasma can be described by an exponential equation of the form:
with
C = drug concentration
C(0) = extrapolated initial drug concentration (see Volume of distribution)
λ = elimination rate constant (see Half-life)
t = time
The elimination rate constant ? can be calculated by fitting the data points during the elimination phase to a single exponential; yielding in this example a ? of 0.34 h-1. An alternative method (see Fig. 3.) consists in plotting the logarithm of the drug plasma concentration as a function of time, which will yield a straight line. The steepness of this line equals –?.
Clinical Implications
In clinical pharmacology, first order kinetics are considered as a « linear process », because the rate of elimination is proportional to the drug concentration. This means that the higher the drug concentration, the higher its elimination rate. In other words, the elimination processes are not saturated and can adapt to the needs of the body, to reduce accumulation of the drug.
95% of the drugs in use at therapeutic concentrations are eliminated by first order elimination kinetics.
A few substances are eliminated by zero-order elimination kinetics, because their elimination process is saturated. Examples are Ethanol, Phenytoin, Salicylates, Cisplatin, Fluoxetin, Omeprazol.
Because in a saturated process the elimination rate is no longer proportional to the drug concentration but decreasing at higher concentrations, zero-order kinetics are also called “non-linear kinetics” in clinical pharmacology.
Terminology and properties
| Elimination kinetics | First order | Zero order |
| Curve in the plasma concentration vs. time plot after i.v. bolus | Exponential decay (Fig. 2) | Linear (Fig. 1) |
| Curve in the log plasma concentration vs. time plot after i.v. bolus | Linear (Fig. 3) | Non-linear |
| Relation between elimination rate and drug concentration | Elimination rate is proportional to drug concentration | Elimination rate saturates with higher drug concentration< |
| Term in clinical pharmacology | Linear kinetics | Non linear kinetics |
| Concerns | 95 % of drugs, at therapeutic concentrations | The remaining 5 %, and ethanol |